I have been studying the neurobehavioral aspects of food and drug reinforcement for the past 5 years (read more about it on my profile page). This involves using rats to mimic basic human behaviors surrounding food and drug intake. I then manipulate various neurotransmitter systems by using drugs and observe the effects this manipulation has on the behaviors I am interested in.
What’s important to this type of research is that we constantly challenge and evaluate the validity of using these animal models to study complex human diseases and disorders. Validity can be divided into several categories, but I’m going to focus on two in particular and relate them to an animal model of binge food intake. These two types of validity are predictive validity and construct validity:
- Predictive validity, when comparing animal research to human research, refers to the ability for some measure of animal behavior to predict a human behavior.
- Construct validity in the case of comparing animal studies to human studies is the degree to which the animal behavior accurately reflects a human construct.
In psychology, a construct refers to things like intelligence, motivation, and knowledge; things that are hard to measure, observe, and operationalize (so that we could measure and study it).
Predictive validity and construct validity are important for preclinical studies (that’s before humans get involved) because they aid in determining what we can reasonably infer or conclude from data. Sometimes both are present, sometimes one, and sometimes none at all (the last one is obviously not very useful).
Here’s where I need YOUR feedback: I’m interested in discussing the validity of a study by Cifani et al. (2009).
In this work, the researchers tested the effects of FOUR DIFFERENT FDA approved drugs on a rat model of caloric restriction and stress-induced binge eating. In this study, the rats got to binge on a delectable mixture of nutella and rat chow = Yum!
They were testing these drugs is to validate their model as useful for predicting the efficacy of potential pharmaceuticals for the treatment of binge eating.
The authors divided the rats into four groups:
- non-restricted and not exposed to stress (NR + NS)
- restricted and not exposed to stress (R + NS)
- non-restricted and exposed to stress (NR + S)
- restricted and exposed to stress (R + S)
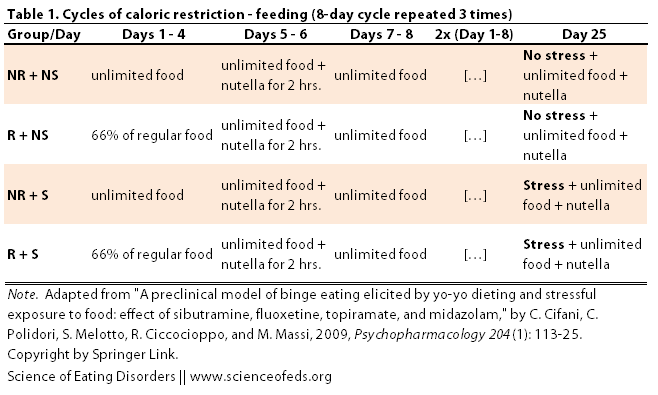
The rats then underwent an 8-day cycle that was repeated 3 times (see table below). The restricted groups (R + NS and R + S) got 67% of the regular food intake for the first four days of of the cycle whereas the non-restricted groups had unlimited food access during those days. On days 5 and 6, all animals got a yummy nutella mix for 2 hours in addition to their regular food. One days 7 and 8, all groups had unlimited access to food.
After the third cycle, the stressed groups (NR + S and R + S) were shown the nutella container and could smell the nutella, but could NOT access it. This procedure was argued to evoke a mild stress response akin to exposure to a “banned” food in humans, restraining from eating that taboo food, and then subsequently bingeing on it.
After this “stress” experience, all animals were exposed to a test session where they received free access to their regular food and the nutella paste. During the test session, ,the authors looked at the effects of this 24-day cycle on nutella/regular food intake, body weight, and stress hormone levels among the different groups.
WHAT DID THEY FIND?
- The restricted and stressed (R + S) group showed the highest overall intake of the nutella paste
- Both groups exposed to stress (R + S and NR + S) had higher stress hormone levels
- There were no differences in body weight among the four experimental groups
The body weight and stress hormone measures showed that it is the combination of food restriction and stress that contributed to binge eating in this model.
Next, the authors assessed the effects of four different drugs on binge eating in the four different rat groups. Two drugs were SSRIs: Fluoxetine (Prozac®) and Sibutramine (Reductil®, also a selective norepinephrine reuptake inhibitor), one was Topiramate (Topomax®, an anti-epileptic drug with some indications of reducing binge eating), and the final drug was the anxiolytic drug Midazolam (Ipnovel®, a benzodiazepine).
MAIN RESULTS
- Both SSRIs (Fluoxetine and Sibutramine) reduced food intake in all rat groups.
- The anti-epileptic Topiramate reduced binge eating ONLY in the restricted and stressed (R + S) group.
- The anxiolytic Midazolam had no effects.
Continuously evaluating the efficacy of animal models is immensely important to the field of drug development. Those of us at the “bench” need to engage in cross-talk with those at the “bedside” (i.e., clinic) to ensure that we have the most accurate interpretations of our outcomes and what their potential implications for the ED field may be.
And here’s where my questions for you come in:
1) SSRIs have mixed-efficacy in ED patients, but certainly some individuals DO benefit. However, is this model sensitive enough to have any predictive validity for novel therapeutics?
2) The use of SSRIs reduced food intake in ALL groups, not just those that were exposed to stress and food-restriction. Thus, could this drug act by curbing appetite in general? Wouldn’t this be the OPPOSITE of what we would want in a drug to treat eating disorders? Specifically, could the use of SSRIs potentially reinforce continued food restriction among a subset of eating disorder patients?
3) What (construct) is being measured here? Do you think this model adequately reflects binge eating in humans? The stressor is very food-specific, which may not correctly model the multitude of different precipitators of binge eating. Do you think this limits what we can conclude about the effects of the drugs on binge eating?
So, let me know what you think in the comments! And, of course, don’t hesitate to ask questions if anything is unclear.
References
Cifani, C., Polidori, C., Melotto, S., Ciccocioppo, R., & Massi, M. (2009). A preclinical model of binge eating elicited by yo-yo dieting and stressful exposure to food: effect of sibutramine, fluoxetine, topiramate, and midazolam Psychopharmacology, 204 (1), 113-125 DOI: 10.1007/s00213-008-1442-y
I’m confused as to what is trying to be treated exactly, is it binge-eating by itself or comorbid with another ED? But from what I understand topiramate can also be used for weight loss, so it works in treatment for binge-eating but for AN/BN/Sub-threshhold isn’t that bad? Also, when I was obsessed with restricting I was on SSRI’s for my depression and I’m wondering if that could have added to my weight loss. I’m pretty sure that EDs can be comorbid with depression. Also, I don’t think rats have a way of stopping themselves from binging, while those who are restricting can find ways around binging (I don’t know if this is relevant but it came to mind).
I am not a scientist, please don’t judge me.
Hi Cris!
I would NEVER judge your input! Honestly, this is EXACTLY the type of insight that we need, and I find your comments very helpful. I agree with you that the authors are not explicit in whether they are trying to treat binge eating itself (i.e., “binge eating disorder”, according to the DSM V), or whether they want to develop a model for bulimia nervosa. The authors claim that this can be a model for both, which I think is a huge overstretch of their model.
In regards to the rats, they will stop themselves from bingeing, probably better than humans will. I’ve found they are a bit more responsive to interoceptive cues than we are. Yet another potential concern if you are trying to model the human condition.
What we can see from this model is that stress and food restriction can additively contribute to binge eating (probably a “no brainer” statement there). These drugs may be able to aid in suppressing that urge. However, as you acutely pointed out, there could be potential NEGATIVE side effects of using drugs that may promote weight loss and appetite suppression (such as SSRIs) and we should, of course, be aware of that potential in prescribing these medications.
Thanks so much for your input! I really appreciate it!
I was looking over a cartoon that explained a study of addiction in rats this morning – I didn’t get all the way through it as I was called away. But basically it was showing how outside factors do affect addictive behaviors – they got the rats addicted to morphine while keeping one group in a ‘rat city’ of cages, and the other group in a ‘rat playground’ that was really nice for them to be in and play in. They found (at least in the part I was up to) that the rats in the playground became less addicted to the morphine, and resisted it more strongly.
Definitely the stressor of withholding the food would make a huge difference. It seems that deprivation or even the fear of deprivation can often lead to bingeing and food hoarding in humans, I would not be surprised that it does in rats too, given that they also need to eat to remain alive. I think rats would make good models for biologically driven or neurologically driven ED or food behaviors but they wouldn’t really give us much of an idea of other factors that human beings do come up against eg cultural pressures, weight shaming etc.
You are totally right about exposure to the “rat playground” and the likelihood of morphine taking in these animals (if you ever want to check out more of these studies, you can search the keyword “enriched environment”).
I like that you point out that there are parallels between some of the basic neurologial drivers of “metabolic need” in rats and humans. Regardless of the cognitive influences, food deprivation in both rats and humans will increase drives to “binge” and “hoard” and these factors interact with higher-level cognitions in ED. That knowledge helped me feel less guilty when I would sometimes binge on fatty foods just because they were available, despite my efforts to eat healthy. The cue of the food itself was enough to drive that behavior, sort of like the rats.
Thanks for the input!